Baermed Solutions
Commercial use of Baermed products
Availability of Baermed products and treatment methods for a wide range of patients
Baermed Solutions deals with the commercial use of all products and treatment methods under development in the field of biomedical mini-organs. These include the HEPADUA® Mini-Liver Implant for patients with chronic liver disease and cirrhosis, as well as products and treatment methods based on the unique Matrix technology. The global market potential of all Baermed products is very high.
As soon as clinical research with the current studies on the treatment of severe, chronic liver cirrhosis with liver cell matrix implants is successfully completed, the results will be made available to a broad group of patients.
Baermed Bauchmedizin AG and Baermed Liver Cell Technologies Pte. Ltd. are in discussions with various international partners for a market launch of the treatment method with liver cell matrix implants (HEPADUA® Mini-Liver Implant).
Biomedical mini organs
Biomedical mini-organs and cell matrix implants.
Baermed Group is focused on the development of biomedical mini-organs and cell matrix implants as well as innovative treatments for chronic liver diseases and liver cirrhosis.
The medical products and treatment methods are based on the results of basic scientific research and clinical research conducted by the Baermed Group and its research partners.

Products and treatment methods
HEPADUA® Mini-Liver Implant, Anti-Adhesion Matrix®, Fibroblast Matrix®.
HEPADUA® Mini-Liver Implant
Baermed’s current focus is on the research and replicable use of a mini-liver implant. The HEPADUA® Mini-Liver Implant is an innovative treatment method for chronic liver disease or a severely damaged liver. The implant consists of an autologous liver and pancreas cell mixture that is seeded onto a specially surface-treated carrier substance (called matrix) and implanted in patients.

Further product developments
Other biomedical products are in the development phase, such as an anti-adhesion matrix and a fibroblast matrix for hernia repair. The products are in a preclinical phase, which will be completed in the near future.
Anti-Adhesion Matrix®
Baermed is developing a two-layer matrix implant (anti-adhesion matrix) with a hydrophilic top layer facing the peritoneum and a hydrophobic layer facing the abdominal organs. The first layer is placed near the wound surface and prevents the formation of postoperative serosal fluid, while the lower layer prevents adhesions to intra-abdominal organs.
The first animal tests have shown promising results (patent pending). The applications for the clinical trials for the Anti-Adhesion Matrix® are currently being drafted and will be submitted to the relevant ethics committee in Indonesia in due course.

Fibroblast Matrix®
Baermed is developing a sponge-like matrix with implanted autologous fibroblasts (fibroblast matrix) for the treatment of hernias and incisional hernias. The first animal experiments have shown that the implanted fibroblasts are numerically larger than when an empty matrix without fibroblasts is implanted.
The additional fibroblast cells form a larger layer of fibroblasts that also migrate into the wound and produce collagen to enhance normal wound healing (patents pending). The applications for the clinical trials for the Fibroblast Matrix® are currently being drafted and will be submitted to the relevant ethics committee in Indonesia in due course.

HEPADUA® MINI-LIVER IMPLANT
Novel treatment method for chronic liver disease and liver cirrhosis
Baermed has developed the HEPADUA® Mini Liver Implant, a patented cell regeneration procedure that offers patients with chronic liver disease or a severely damaged liver (cirrhosis) a better quality of life.
High market potential for HEPADUA® Mini-Liver Implant
MILLIONS OF PEOPLE WITH CHRONIC LIVER DISEASE OR CIRRHOSIS DIE OR SUFFER NEEDLESSLY
159 million people suffer from liver cirrhosis
Liver cirrhosis due to hepatitis B and C virus, alcoholic liver disease, and nonalcoholic fatty liver disease are a worldwide public health problem. 159 million people with liver cirrhosis worldwide would need a total organ transplant. Today, only liver transplantation can cure the symptoms of severe or end-stage cirrhosis caused by these underlying diseases.
▶︎ Complete liver organ transplantation is the only option for treating liver cirrhosis.
Worldwide shortage of liver transplant organs
Unfortunately, there is a shortage of organs for organ transplantation worldwide. Living donor liver transplantation, partial liver transplantation and other methods have not changed this problem. On the contrary, the number of non-obese alcoholic liver diseases leading to cirrhosis will continue to increase, exacerbating the problem. Read more >
Ideas with extracorporeal devices that were supposed to remove the toxic substances produced in liver failure and function like dialysis in end-stage renal disease (ESRD) have not met the high expectations. Therefore, except for liver organ transplantation, there is no cure for cirrhotic liver disease.
The worldwide shortage of transplantable organs leads to the sad effect that many patients do not make it onto a transplant list and die while waiting for a transplantable liver organ.
For every patient who makes it onto a liver transplant list, there are about 10 to 20 patients who do not make it onto the list because they are not eligible for an organ transplant due to another disease.
▶︎ Treatment at an earlier stage can save lives.
Organ transplantation is culturally not accepted or too expensive
In several countries (e.g., Asia), there are no widely available liver transplant programs due to cultural understanding. In addition, patients can rarely afford liver transplantation due to their low income.
▶︎ The treatment method with an implant is usually compatible with cultural understanding and the cost of treatment is manageable, so that patients with low incomes can also afford a HEPADUA® Mini-Liver Implant. An implant costs 20% of a full organ transplant.
HEPADUA® Mini-Liver Implant gives hope
The HEPADUA® Mini-Liver Implant developed by Prof. Dr. med. Hans U. Baer and his research team gives new hope to patients suffering from chronic liver disease or cirrhosis.
Early treatment with the HEPADUA® Mini-Liver Implant can save lives and sustainably improve quality of life. On the one hand, the implant can demonstrably alleviate symptoms, increase life expectancy and, on the other hand, serve as a useful stopgap for patients who are already on a waiting list for a liver transplant. The mini-liver can be implanted repeatedly.
Advantages of a HEPADUA® Mini-Liver Implant
159 million people worldwide suffer from cirrhosis of the liver, which leads to death:
► A HEPADUA® Mini-Liver Implant can save the lives of hundreds of thousands of people.
There is a shortage of donor liver organs worldwide:
► A HEPADUA® Mini Liver Implant uses the patient’s own liver cells and enables cell regeneration. Timely treatment with the implant can save lives and sustainably improve the quality of life. A complete liver transplant can thus be bridged or delayed.
Organ transplantation is culturally unacceptable or too expensive:
► HEPADUA®Mini Liver Implant treatment is culturally accepted and costs 20% of a full organ transplant.
Function and effect of the HEPADUA® Mini-Liver Implant
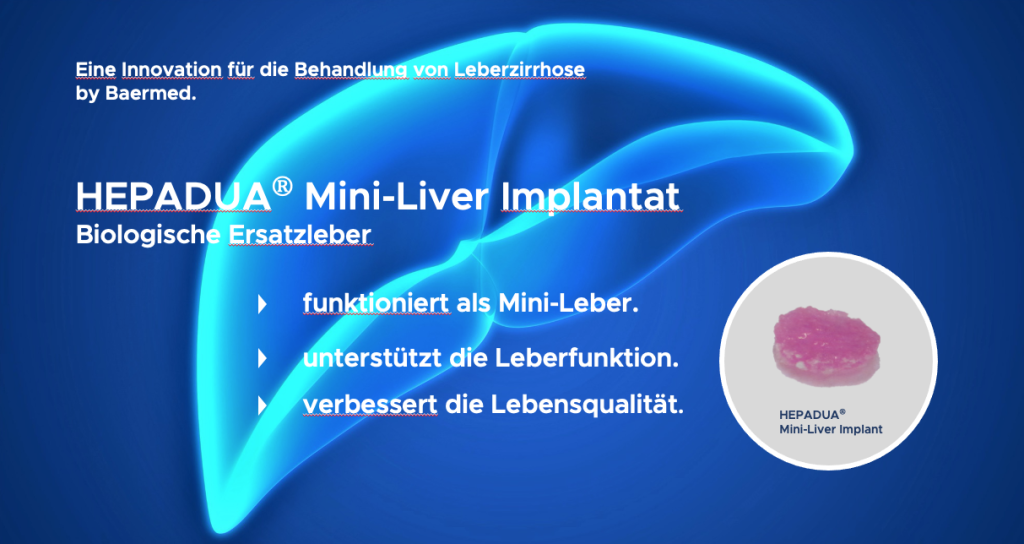
The HEPADUA® Mini-Liver Implant is an innovative, biological liver substitute and is considered a breakthrough in the treatment of liver cirrhosis.
The HEPADUA® Mini-Liver Implant…
- works like a mini liver
- supports liver function
- Improves the quality of life

The HEPADUA® Mini-Liver Implant is implanted subserosally in the mesentery of the small intestine and connected to the portal vein and thus to the liver via the splanchnic vessels. This method allows the implant to “communicate” with the liver and support liver function.
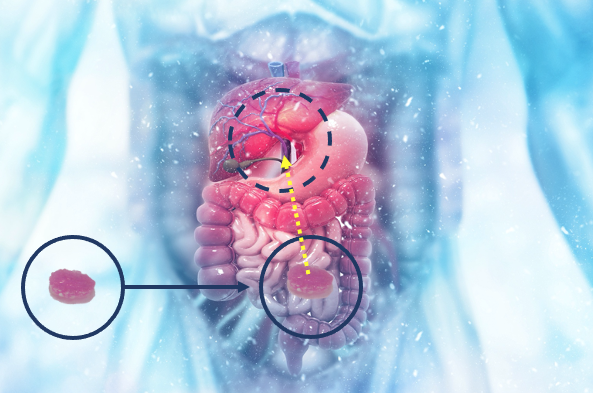
HEPADUA® implant procedure
A breakthrough in the treatment of chronic liver disease and liver cirrhosis
The innovative HEPADUA® technology enables the implantation of the patient’s own liver cells by means of a matrix, which functions as a “mini-liver”.
Timely treatment with the HEPADUA® Mini-Liver Implant can save lives and sustainably improve patients’ quality of life in cases of chronic liver disease and cirrhosis.
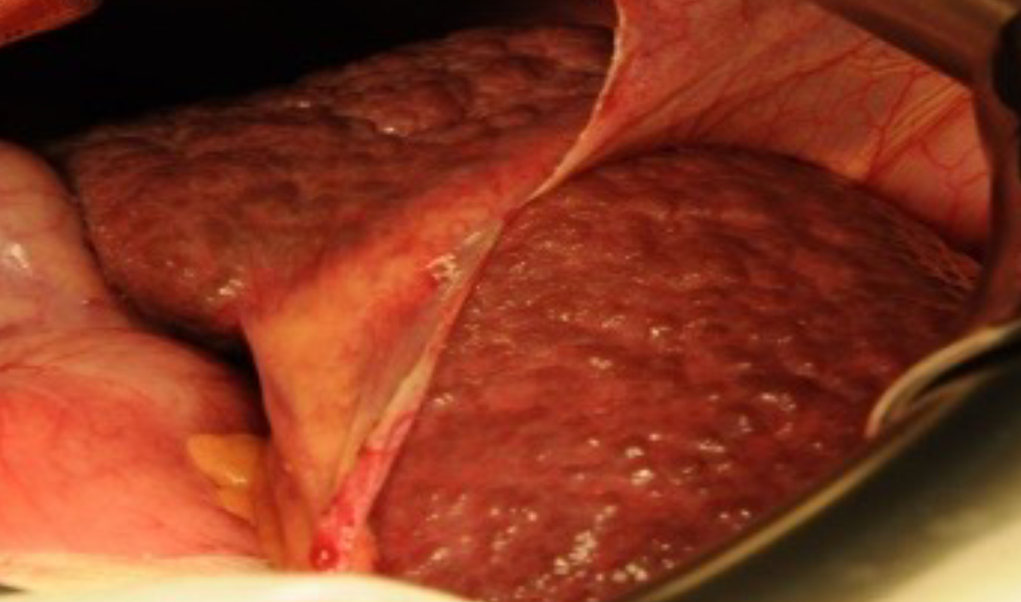
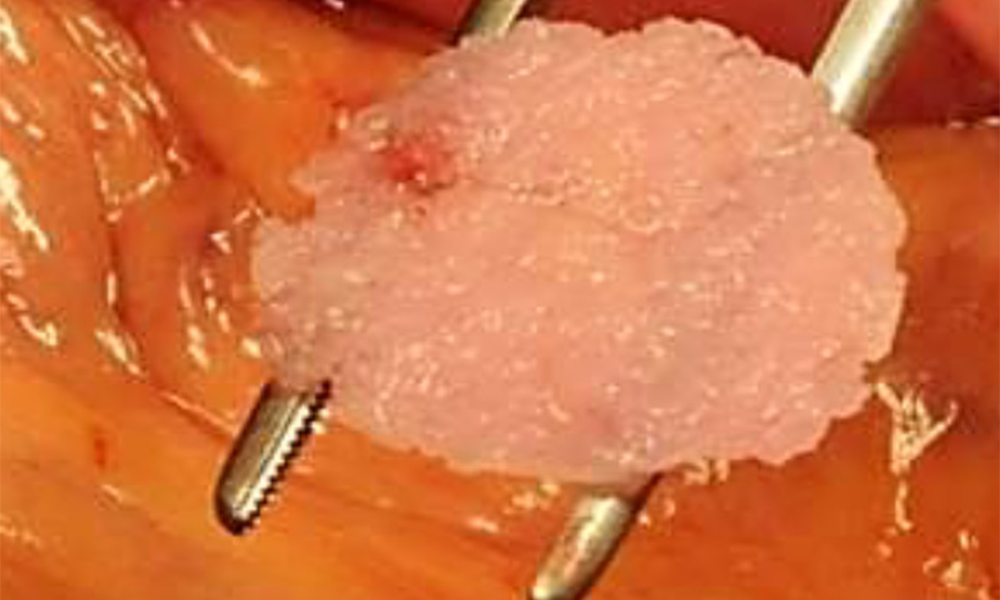
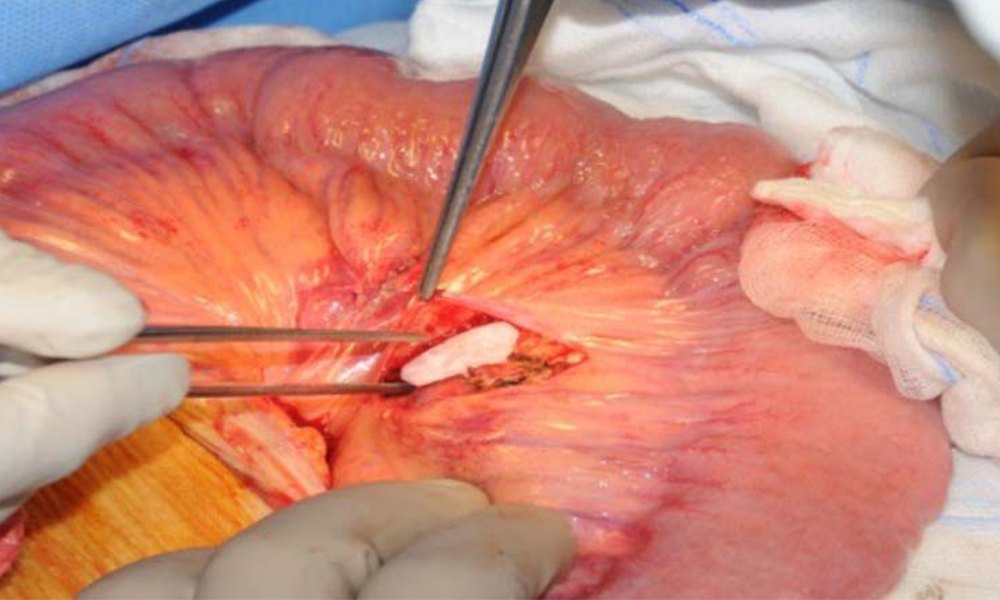
Hepatocyte Matrix Implant Method
Three-dimensional PLLA matrices are fabricated as a support material or spongy scaffold to facilitate effective settlement of seeded cells of different types, in the case of mini-liver of hepatocytes and pancreatic cells. Most of the seeded cells adhere to the PLLA base material, grow, signal, and interact with other cells while maintaining the cells’ metabolic function. Read more >
The PLLA scaffold is biocompatible and self-biodegradable. It is produced in the form of a button-like matrix with a diameter of 20 mm. The individual liver and pancreas cells are obtained from tissues removed from the patient. They are mixed (the ratio of cells is evaluated according to cell counting) and arranged to be seeded onto the matrices at a concentration of 1-2 million cells per matrix and incubated for at least 48 hours at 37 °C and 5% CO2.
After incubation, the remaining cells that do not adhere to the matrices are counted and cell viability is assessed, again using the hemocytometer under inverted microscopy with the trypan blue exclusion method. The ratio of hepatocytes to pancreatic cells is patent.
Cell growth can be promoted by growth factors or growth factors that stimulate hepatocytes can be used without implanting pancreatic cells.



HEPADUA® Matrix
The unique HEPADUA® matrix serves as an active “platform” for interaction, signal transduction and cell growth of the implanted liver cells.
HEPADUA® Cell Matrix Culture
The research team has developed an innovative cell matrix culture method for the selection and preparation of liver cells that allows the highest possible number of implanted cells.
HEPADUA® Mini-Liver Implant
After 3 days, the HEPADUA® Mini-Liver Implant can be removed from the cell matrix culture and implanted in the patient.
At a glance
HEPADUA® treatment method
- Simple, short and safe treatment method
- Patients are donors of their own liver cells.
- The preparation of the cell culture takes 3 days.
- The recovery takes only 14 days.
- Immunosuppressants are not required.
HEPADUA® Effect
- Optimizes liver function
- Stimulates the repair of the liver*
- May reduce liver cancer risk*
- Enables participation in society
- Prolongs life
- Improves the quality of life
*) still has to be proven in clinical studies
Information for investors
Baermed Bauchmedizin AG is currently privately owned and privately financed. The Baermed Group is open to additional investments in order to strengthen and expand its research and development activities and successfully make its innovative products and treatment methods available to a broad range of people.
Investors, whether private or institutional, will hold shares in Baermed Liver Cell Technologies Pte. Ltd. with worldwide distribution rights for the Hepadua® Mini-Liver Implant. Baermed is confident and convinced that an investment is extremely attractive.
Please contact Drs. Karin van Zuilen or Dr. Coert Huijbregsen for further information (see also “Contact for Investors”).
Management team commercial use and research
Management team commercial use Baermed products
Drs. Karin van Zuilen, Pharmacist, and Coert Huijbregsen, MD, lead Senz Strategy Consulting, based in Malaysia, focusing on strategic consulting in the life sciences industry.
They are the representative of Baermed Liver Cell Technologies Pte. Ltd. based in Singapore and responsible for the commercial exploitation of the Baermed product and treatment methods (biomedical mini-organs and cell matrix implants).

Managing Director Senz Strategy Consulting
Consultant of Baermed Liver Cell Technologies Pte. Ltd, Singapore

Senior Consultantt Senz Strategy Consulting
Consultant of Baermed Liver Cell Technologies Pte. Ltd, Singapore
Research management team

Specialist FMH for Visceral Surgery, Master of Applied Science, Klinik Hirslanden Zürich, Chairman of the Executive Board of Baermed Bauchmedizin AG, International Director and Head of Research of the Tarumanagara Human Cell Technologies Laboratory (THCT Lab)

Head of Tarumanagara Human Cell Technologies Laboratory (THCT Lab) and Senior Researcher of Faculty of Medicine, Tarumanagara University (UNTAR)
Organization commercial use Baermed products
BAERMED SOLUTIONS
Commercial use of biomedical mini-organs and cell matrix implants.
Overall operational responsibility
Drs. Karin van Zuilen and Dr. Coert Huijbregsen
Baermed Liver Cell Technologies Pte. Ltd.
Simon Hu
Chairman of the Board of Directors
___
Singapore
Baermed Human Cell Technologies
Ltd.
Lisbeth Hasler
Chairman of the Board of Directors
___
Principality of Liechtenstein
Innovative products
Baermed has developed a number of innovative products and treatment methods in the field of biomedical mini-organs and cell matrix implants that show great market potential.
These products will be made available to a wide range of patients after market approval. The launch of the products and treatment methods will be managed by Baermed Liver Cell Technologies Pte. Ltd. in Singapore.
The first product expected to be commercialized is the HEPADUA® Mini-Liver Implant, which will be licensed to hospitals and will enable the treatment of thousands of patients with liver cirrhosis.
HEPADUA® can save lives
The HEPADUA® Mini-Liver Implant is considered a breakthrough in the treatment of chronic liver disease and cirrhosis.
HEPADUA® Mini-Liver Implant . . .
- functions as a mini liver.
- supports liver function.
- improves the quality of life.
Global market potential of HEPADUA® for the treatment of liver cirrhosis
There is a huge market of liver cirrhosis patients who can only be treated symptomatically or are on the waiting list for a full liver organ transplant.
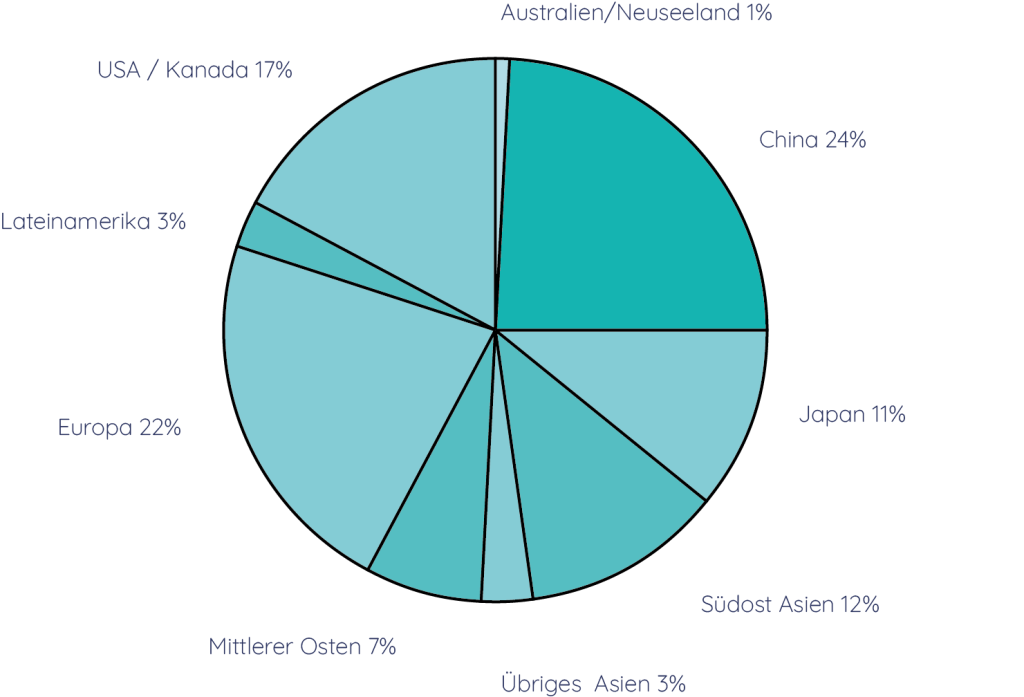
The global market potential for the HEPADUA® Mini-Liver Implant is in the order of USD 25 billion. The market shares are distributed as shown in the chart below.
Milestones HEPADUA® Mini-Liver
- 2002: Collaboration with R.S. Gading Pluit
Start of cooperation with the private hospital group R.S. Gading Pluit in Jakarta, Indonesia.
- 2005: First HEPADUA® Mini-Liver Implant
First HEPADUA® Mini-Liver Implant in a patient with liver cirrhosis by Prof. Hans U. Baer, MD.
- 2009: First Phase I clinical trial in Germany
This study with 57 patients in Germany by Priv. Doz. A. Schwarz, MD, showed encouraging results.
- 2010: Foundation of Tarumanagara Human Cell Technologies Laboratory, Jakarta
Establishment of the Tarumanagara Human Cell Technologies Laboratory (THCT Lab) at Tarumanagara University (UNTAR) in Jakarta, Indonesia. The THCT Lab specializes in research of biomedical mini-organs and tent matrix implants.
- 2018: HEPADUA® Phase I clinical trial in Jakarta.
Successfully completed HEPADUA® Phase I clinical trial with 11 patients in Jakarta, demonstrating the feasibility of the treatment method in liver cirrhosis.
The study was conducted under the direction of Prof. Dr. med. Hans U. Baer in collaboration with Dr. dr. Barlian Sutedja, Sp. B., , Dr. Peter Limas and Dr. Suryadi The, Sp. B., R.S. Gading Pluit Private Hospital, and Dr. dr. Andri Sanityoso SpPD KGEH, Liver Clinic Prof. Ali Sulaiman.
The study was reviewed and approved by the Ethical Committee of the R.S. Gading Pluit Private Hospital, headed by Prof. Ronokusumo Sjamsuhidajat MD. - 2022: HEPADUA® Phase II clinical trial in Jakarta
Approval by the Ethics Committee of the Ministry of Health of the Republic of Indonesia for a HEPADUA® Phase II clinical trial in 32 patients in Jakarta to demonstrate the efficacy of the HEPADUA® Mini-Liver Implant in liver cirrhosis.
The clinical trial is being conducted under the direction of dr. Barlian Sutedja, Sp. B. in collaboration with, Prof. Dr. med. Hans U. Baer, Dr. Peter Limas and Dr. Suryadi The, Sp. B., R.S. Gading Pluit Private Hospital, and Dr. dr. Andri Sanityoso SpPD KGEH, Hati Clinic Prof. Ali Sulaiman. The study was reviewed and extended by the Ethical Committee of the R.S. Gading Pluit Private Hospital, headed by Prof. Ronokusumo Sjamsuhidajat MD. The extension was necessary due to the Covid 19 pandemic.
Proof of Concept HEPADUA® Mini-Liver
✔︎ 2005: First HEPADUA® Mini-Liver Implant by Prof. Dr. med. Hans U. Baer
First treatment of a patient with the HEPADUA® Mini-Liver Implant as an individual healing trial.
✔︎ 2009: First clinical study with 57 patients in Germany by Priv.-Doz. Dr. med. A. Schwarz
The study results concerning the feasibility and efficacy of the HEPADUA® Mini-Liver Implant were very encouraging. The findings enabled optimization of the HEPADUA® technology.
Literature: A Schwarz, T Lindl, C Höhneke, M Stange, W Pieken. Human Autologous Liver Cell Transplantation for the Treatment of Cirrhosis. The Internet Journal of Gastroenterology. 2009; 10:1.
✔︎ 2018: Phase I clinical trial with 11 patients in Indonesia led by Prof. Hans U. Baer, MD.
The study conducted at the R.S. Gading Pluit Private Hospital in Jakarta has demonstrated the feasibility of the HEPADUA® Mini-Liver Implant in liver cirrhosis. Patients generally experienced an improvement in their quality of life. These results enabled the projection of a subsequent Phase II study.
Literature: Baer HU, Hendrawan S, The S, Lelosutan SAR, Salim G, Lindl T, , et al. IntracorporealAutologousHepatocyte Matrix Implantforthe Treatment of ChronicLiverDisease: A Modified Clinical Phase I Study. World J SurgSurgical Res. 2018; 1: 1067
✔︎ 2022: Phase II clinical trial with 32 patients in Indonesia led by dr. dr. Barlian Sutedja, Sp. B.
The study began in March 2022 at the R.S. Gading Pluit Private Hospital in Jakarta and is designed to prove the efficacy and potential side effects of the HEPADUA® Mini-Liver Implant in chronic cirrhosis. A positive study result is the prerequisite for official registration of the HEPADUA® treatment method.
Market launch of the HEPADUA® Mini-Liver
The market launch of the HEPADUA® Mini-Liver Implant in Asia is planned in 2024/25.
Investment incentives for HEPADUA® Mini-Liver
- 1. Saves the lives of a large population group
The lives of hundreds of thousands of people with chronic cirrhosis can be improved and saved with a HEPADUA® Mini-Liver Implant.
Cirrhosis of the liver is one of the leading causes of death worldwide.
- 2. Unique medical technology
HEPADUA® Mini-Liver technology is considered an innovative breakthrough in the treatment of liver cirrhosis.
The treatment method and the procedure are patented.
The Baermed matrix and treatment method developed in-house are unique.
There is no comparable, competitive medical technology on the market.
- 3. High market potential
There is a huge market of liver cirrhosis patients who can only be treated symptomatically or are on the waiting list for a full liver organ transplant.
The global market potential for the HEPADUA® Mini-Liver Implant is in the order of USD 25 billion.
- 4. Proof of concept available
A large number of successful preclinical animal studies and initial clinical trials in patients with liver cirrhosis have demonstrated the feasibility and efficacy of the HEPADUA® Mini-Liver treatment method.
- 5. High ROI
With a steeply rising enterprise value, the return on investment for HEPADUA® Mini-Liver technology looks very promising.
HEPADUA® Mini-Liver – An Innovation for the Treatment of Liver Cirrhosis
Contact for investors
Commercial use Baermed products
Contact us to learn more about HEPADUA® or partnering with Baermed.
Drs. Karin van Zuilen
CEO
Baermed Liver Cell Technologies Pte. Ltd.
Singapore
Dr. Coert Huijbregsen
COO
Baermed Liver Cell Technologies Pte. Ltd.
Singapore