Baermed Research
Research areas
Tissue engineering, mini-organs and cell matrix implants
Baermed Research specializes in tissue engineering and the development of mini-organs and cell matrix implants.
The central goal of Baermed Research is to be able to provide patients with chronic liver diseases with long-term support and help using the method of liver cell transplantation.
“We are very optimistic that with our method of liver cell transplantation – HEPADUA® Mini-Liver – we will find a way to effectively treat patients with liver cirrhosis to improve their quality of life in the long term.”
On behalf of the Baermed Research Partners:
Prof. Dr. med. Hans U. Baer
CEO Baermed Abdominal Medicine and International Director Tarumanagara and Researcher Human Cell Technology Laboratory (THCT Lab) at Tarumanagara University (UNTAR), Head of Research Baermed Group, Hirslanden Clinic Zurich, Switzerland.
Assoc. Prof. Siufui Hendrawan, MD, PhD
Head Tarumanagara Human Cell Technology Laboratory (THCT Lab) at the Faculty of Medicine, Tarumanagara University (UNTAR) in Jakarta, Indonesia.

CEO Baermed Abdominal Medicine, Hirslanden Clinic Zurich, Switzerland; International Director of Tarumanagara Human Cell Technology Laboratory (THCT Lab) at Tarumanagara University (UNTAR), Head of Research Baermed Group.

Head Tarumanagara Human Cell Technology Laboratory (THCT Lab) at the Faculty of Medicine, Tarumanagara University (UNTAR) in Jakarta, Indonesia.
Research spectrum
Basic and clinical research
Baermed Research conducts basic and clinical research in the fields of cell biology, tissue engineering and the development and implementation of matrix carriers for functional mini-organs. Prof. Dr. med. Hans U. Baer, Center for Abdominal Medicine at the Hirslanden Clinic Zurich, is leading the research work.

(l.t.r.) Assoc. Prof. Siufui Hendrawan, MD, PhD, Head Tarumanagara Human Cell Technology Laboratory (THCT Lab); Guna Mahalingam, Founder & CEO Strategic Swiss Partners AG, Partner of Baermed Research; Ursula Weber (†2022), International Consultant and Research Partner, former. Member of the Executive Board and Head of Organization and Administration Baermed; Prof. Dr. med. Hans U. Baer, CEO Baermed – Center for Abdominal Medicine and Visceral Surgery, Head of Research Baermed Group, Hirslanden Clinic, Zurich, and International Director of the Tarumanagara of the Human Cell Technology Laboratory (THCT Lab) at Tarumanagara University (UNTAR).
Basic research
Basic research is conducted at the Tarumanagara Human Cell Technology Laboratory (THCT Lab) at Tarumanagara University (UNTAR) in Jakarta, Indonesia. It was founded in 2010 on the initiative of Prof. Dr. med. Hans U. Baer.
Prof. Dr. med. Hans U. Baer, and his team at the Tarumanagara Human Cell Technology Laboratory (THCT Lab) conduct research in the fields of cell biology, tissue engineering, and the development of matrix carriers for functional mini-organs. He is a member of the Faculty of Medicine, Tarumanagara University (UNTAR) and leads the Tarumanagara Research Center team as International Director and Research Director.
After several years of basic research, Prof. Dr. med. Hans U. Baer, and his team at the THCT Lab achieved a breakthrough in 2018 with the HEPADUA® Mini-Liver Implant project to produce a functional mini-liver.


Clinical research
In addition to basic research, Prof. Dr. med. Hans U. Baer and his team conduct clinical research – based on the findings of basic research – in close cooperation with the R.S. Gading Pluit Private Hospital Group in Jakarta, Indonesia, and with the Liver Clinic Prof. Ali Sulaiman in Jakarta, Indonesia (Liver Cell Clinic). The research collaboration is focused on inpatient, clinical research.
Encouraging research results
The successful research results of the first clinical trial (Phase I) for the treatment of severe, chronic liver cirrhosis with HEPADUA® Mini-Liver Implant,encouraged Prof. Baer and his team to intensify clinical research starting in 2018.
Interruption of research work during the COVID-19 pandemic
During the COVID pandemic in Indonesia, clinical trials had to be interrupted and laboratory work had to adapt to the new situation. Tarumanagara Human Cell Technology Laboratory (THCT Lab) temporarily stopped all of the research work and worked full time as a COVID-19 testing center in collaboration with Jakarta Public Health Office. The laboratory provides COVID-19 PCR and rapid antigen testing In addition to the laboratory staff, up to 15 volunteers took care of the large number of samples. The laboratory could process up to 500 samples per day.

Continuation of research after the COVID-19 pandemic.
In March 2022, the interdisciplinary team resumed clinical research work and started to continue the second clinical trial (Phase II) for the treatment of severe, chronic liver cirrhosis with HEPADUA® Mini-Liver Implant.
Research Organization
Basic and clinical research
The 3-pillar principle of Baermed Research
Baermed Research is organizationally based on three pillars:
Pillar 1:
Basic Research – Tarumanagara Human Cell Technology Laboratory
Tarumanagara Human Cell Technology Laboratory in Jakarta (THCT Lab) at Tarumanagara University (UNTAR) in Jakarta, Indonesia. Field of research: Research Center for Biomedical Tissue Engineering and Basic Research (HEPADUA® Mini-Liver Implant)
Pillar 2:
Basic and clinical research – Baermed Abdominal Medicine and Visceral Surgery
Baermed – Center for Abdominal Medicine and Visceral Surgery Hirslanden Clinic in Zurich, Switzerland. Research Director and International Director Tarumanagara Human Cell Technology Laboratory (THCT Lab): Prof. Dr. med. Hans U. Baer, Master of Applied Ethics
Pillar 3:
Clinical Research – R.S. Gading Pluit Private Hospital Group and Liver Clinic Prof. Ali Sulaiman
R.S. Gading Pluit Private Hospital Group and Liver Clinic Prof. Ali Sulaiman in Jakarta, Indonesia. Research area: inpatient, clinical research and clinical hepatology research.
Organization chart of Baermed Research
BAERMED RESEARCH
Organization
Pillar 1:
BASIC RESEARCH
Tarumanagara Human Cell Technology Laboratory
(THCT Lab)
Research Center for Biomedical Tissue Engineering and Basic Research
___
Tarumanagara University (UNTAR)
Jakarta | Indonesia
Pillar 2:
BASIC RESEARCH AND
CLINICAL RESEARCH
Baermed
Center for Abdominal Medicine and Visceral Surgery Hirslanden Clinic
Prof. Dr. med. Hans U. Baer
Research Director and International Director Tarumanagara Human Cell Technology Laboratory.
(THCT Lab)
___
Hirslanden Clinic
Zurich | Switzerland
Pillar 3:
CLINICAL RESEARCH
R.S. Gading Pluit Private Hospital Group &
Liver Clinic Prof. Ali Sulaiman
Research area: inpatient, clinical research and clinical hepatology research.
___
both institutes
Jakarta | Indonesia
Current research project
HEPADUA® Mini-Liver Implant
Novel treatment method with a functional tent matrix implant for liver cirrhosis.
Research partner
Tarumanagara University (UNTAR) and Tarumanagara Human Cell Technology Laboratory (THCT Lab), Baermed Abdominal Medicine, R.S. Gading Pluit and Hati Clinic Prof. Ali Sulaiman.





Tarumanagara University Jakarta (UNTAR)
Tarumanagara University (UNTAR) is a university in Jakarta, Indonesia, and one of the oldest private universities in the country. In 2017, UNTAR received an A accreditation (i.e., excellent) from the National Accreditation Board for Higher Education (BAN-PT).
It has 4 campuses, with Campus I (main campus) and Campus II located in Greater West Jakarta and Campus III in South Jakarta. A future Campus IV, covering 130 hectares and currently under construction, is located in Karawaci (a satellite town about 25 kilometers from West Jakarta) and will become an integrated campus called Tarumanagara City.

Currently, UNTAR is headed by Rector Prof. Dr. Ir. Agustinus Purna Irawan, M.T., M.M., I.P.U., A.E. and Vice Rector Dr. Rasji, S.H., M.H.
The University has eight faculties and one postgraduate program: Faculty of Economics and Business, Faculty of Law, Faculty of Engineering, Faculty of Medicine, Faculty of Psychology, Faculty of Art and Design, Faculty of Information Technology, Faculty of Communication Studies, and Graduate Business School. The university offers bachelor’s and master’s degree programs, as well as doctoral degrees by research.
The Faculty of Medicine, with which the Tarumanagara Human Cell Technology Laboratory (THCT Lab) is affiliated, is currently headed by Dr. dr. Noer Saelan Tadjudin, Sp.KJ.

International partnership
Collaboration between Tarumanagara University (UNTAR), Baermed Abdominal Medicine and Tarumanagara Human Cell Technology Laboratory (THCT Lab).
2010 marks the start of a close collaboration between Tarumanagara University (UNTAR), Baermed Abdominal Medicine and Tarumanagara Human Cell Technology Laboratory (THCT Lab).
At a formal ceremony, the collaboration is sealed by Dr. Gunardi Lie S.H., M.H., Former Chairman of Tarumanagara Foundation (2017-2021 period); Prof. Dr. med. Hans U. Baer, International Director Tarumanagara Human Cell Technology Laboratory (THCT Lab) and Dr. dr. Meilani Kumala, MS., Sp. GK (K), Former Dean Faculty of Medicine (2017-2021 period) Tarumanagara University (UNTAR).

Tarumanagara Human Cell Technology Laboratory (THCT Lab)
The Tarumanagara Human Cell Technology Laboratory (THCT Lab), specializing in cell biology, tissue engineering, and the development of matrix carriers for functional mini-organs, is affiliated with the Tarumanagara University Faculty of Medicine (UNTAR). Baermed co-owns the THCT Lab at Tarumanagara University (UNTAR) in Jakarta, Indonesia.
The head of the Tarumanagara Human Cell Technology Laboratory (THCT Lab) is Assoc. Prof. Siufui Hendrawan, MD, PhD. She leads an experienced and well-coordinated team of 10 employees.

The laboratory consists of two spatially separated units: one for research with human cells and, at a safe distance from it, one for animal cells.
The THCT human cell laboratory has state-of-the-art biosafety cabinets with particle densities below 100 per cm2 in highly sterile rooms. The scientists work in special protective suits to protect themselves from contamination. The laboratory has been certified as Biosafety Level II.
In ante rooms to the highly sterile wing, the equipment for producing the carrier matrices is located. For research with animal cells, the THCT laboratory has facilities for sterile tissue collection and processing of the tissue into single cells.
The THCT Lab’s basic research is currently focused on the HEPADUA® Mini-Liver Implant research project, the development and fabrication of a functional mini-liver.
Prof. Dr. med. Hans U. Baer and his team achieved a breakthrough in 2018 with the HEPADUA® Mini-Liver project to produce a functional mini-liver.








Organization Tarumanagara Human Cell Technology Laboratory (THCT Lab)
The Tarumanagara Human Cell Technologies Laboratory (THCT Lab), founded in 2010 by Prof. Dr. med. Hans U. Baer, has the following organizational structure: Read more >
Institutional Review Board:
Tarumanagara University, Baermed Center for Abdominal Medicine, R.S. Gading Pluit Private Hospital.
International Director and Researcher:
Prof. Dr. med. Hans U. Baer
Scientific Advisory Board:
Dr. dr. Barlian Sutedja, Sp.B, Indonesia, Chair; Prof. Dr. Reto Stocker, Switzerland, Member; Prof. dr. R. Sjamsuhidajat, Sp.B-KBD, Indonesia, Member; Prof. Andreas Serra, Switzerland, Member; Dr. Jörg Knessl, Switzerland, Member
Administrative Advisory Board:
Rector of Tarumanagara University, Jakarta, Chairperson
Dean of the Faculty of Medicine, Tarumanagara University, Member
National Coordinator:
Arief Oetama, MD, MARS
Chief of the THCT Lab:
Assoc. Prof. Siufui Hendrawan, MD, PhD
Institutional Biosafety Committee:
Suryadi, MD; Gatot S. Lawrence, MD, M.Sc., Sp.PA (K)., PhD
Principal Investigators:
Prof. Dr. med. Hans U. Baer; Assoc. Prof. Siufui Hendrawan, MD, PhD
Bio Safety Officer:
Assoc. Prof. Siufui Hendrawan, MD, PhD
Clinical Research Officer:
Erwin Budi, MD
BSL-2 Lab Operators:
Jennifer Lheman, B.Sc, M.Biotek; Nuraeni, B.Sc; Olivia Marcelina, B.Sc, M.Eng.
Lab Engineer and Facility Manager:
Erwin Uliyantoro
Secretariat:
Renantha, B.E
R.S. Gading Pluit Private Hospital and Liver Clinic Prof. Ali Sulaiman Jakarta, Indonesia
Prof. Dr. med. Hans U. Baer and his team – based on the findings of basic research in collaboration with the Tarumanagara Human Cell Technology Laboratory (THCT Lab) – clinical research in close cooperation with the private hospital. R.S. Gading Pluit Private Hospital in Jakarta, Indonesia, and with the Liver Clinic Prof. Ali Sulaiman in Jakarta, Indonesia (Liver Cell Clinic). Research includes inpatient clinical research and clinical hepatology research.

R.S. Gading Pluit Private Hospital Group, Jakarta
Gading Pluit Private Hospital was officially opened on June 7, 2005 and received ISO 9001:2000 certification on June 10, 2006. Since then, Gading Pluit has constantly strived to lead the way by opening new centers that give people easy access to the most advanced healthcare facilities without having to go abroad.

This was proven by the first installation of MSCT-Scan 64 Slice Somatom Sensation Cardiac and MRI 1.5 Tesla Total Imaging Matrix in 2004, which were the latest diagnostic technology in Indonesia at that time. After that, other centers such as GADING CANCER CENTER, HEART & VASCULAR HEALTH CENTER and GADING CRYO CENTER were established.
In 2008, Gading Pluit Hospital opened CYCLOTRON & PET-CT CENTER, the FIRST PET/CT scanning facility in Indonesia, which is officially opened by the Minister of Research and Technology of the Republic of Indonesia DR. Kusmayanto Kadiman.
The most recent facility was RADIOTHERAPY on April 16, 2013, which changed the name GADING CANCER CENTER to GADING INTEGRATED CANCER CARE (GICC). Thus, all cancer treatment facilities could be consolidated at Gading Pluit Hospital.
“Our vision is to become the most trusted hospital you can be proud of. Therefore, Gading Pluit Hospital is dedicated to providing professional service of international standard through professional and innovative teamwork based on love and care for our fellow human beings.”
In 2017, Gading Pluit Hospital was awarded as a “PARIPURNA” by Komisi Akreditasi Rumah Sakit (KARS).
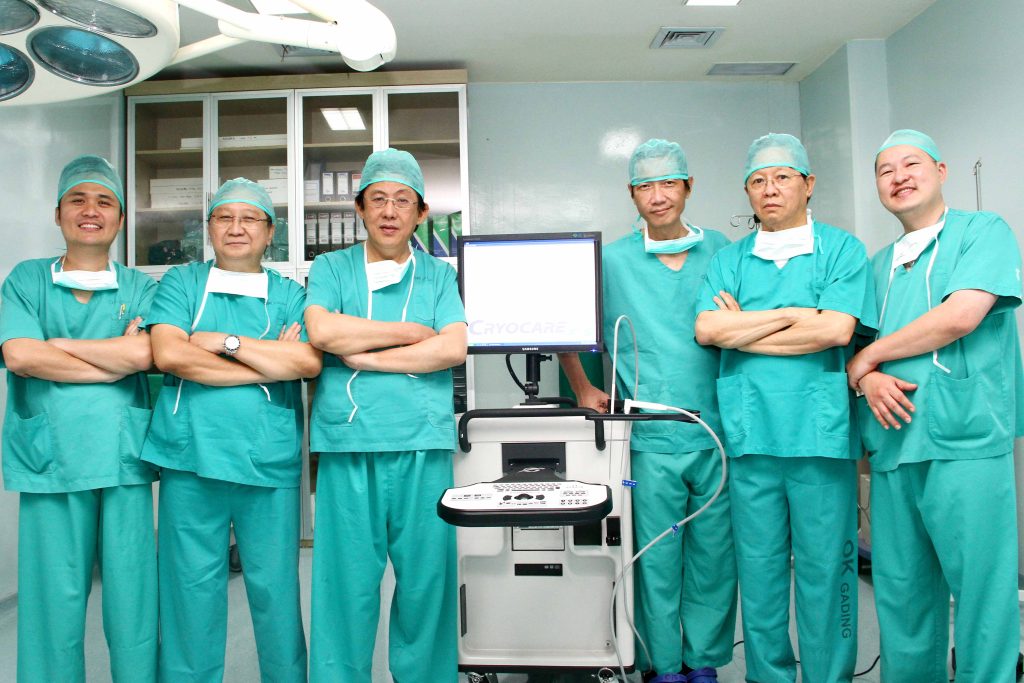
Baermed Clinic Jakarta at R.S. Gading Pluit Private Hospital
In order to provide you with an international standard of quality, Gading Pluit Hospital collaborates with the Baermed Center for Abdominal Surgery and Visceral Surgery at the Hirslanden Clinic in Zurich, Switzerland. For this purpose, each case is examined by the surgeons of Gading Pluit Hospital with the involvement of internists, gastroenterologists, oncologists and intensive care specialists.
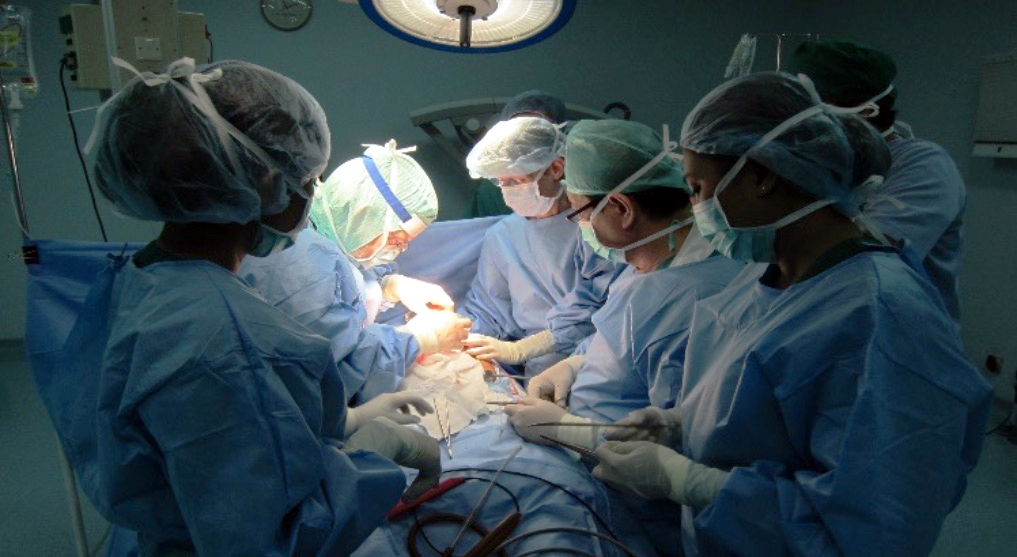
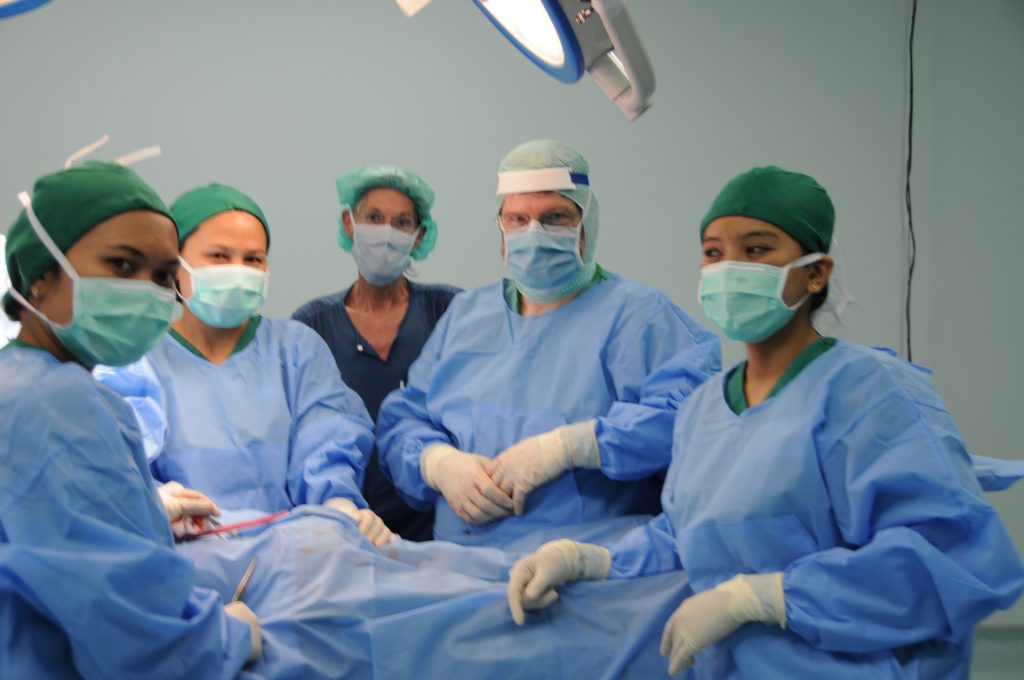
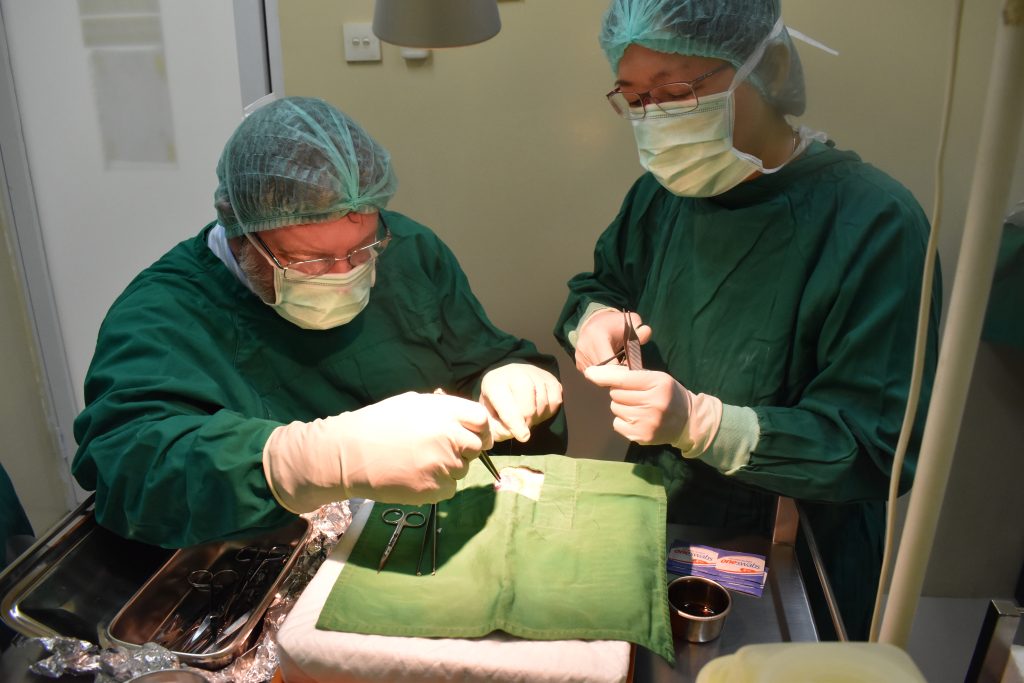
Prof. Dr. med. Hans U. Baer, visits Indonesia regularly and performs teaching surgeries with a team of qualified Indonesian abdominal surgeons at Gading Pluit Hospital and other locations, including specialized hepatobiliary and pancreatic surgery, abdominal oncologic surgery, and complex abdominal surgical cases.
The best approach for each individual patient is discussed between the Indonesian team and Prof. Baer in Switzerland via video link. If necessary, other specialists in Switzerland are called in. In this way, preoperative treatment, further clarification and planning of a possible operation can take place without loss of time. The actual surgeries can be performed either by the Indonesian surgeons or, in the case of very demanding surgical expertise, in collaboration with the Swiss surgeon.
This collaboration is accredited and supported by Persatuan Bedah-Endolaparoscopy Indonesia (PBEI/Indonesian Gastro-Endolaparoscopic Association).
Liver Clinic Prof. Ali Sulaiman, Jakarta
Liver Clinic Prof. Ali Sulaiman is a specialized clinic for the treatment and care of all types of liver diseases. The clinic offers comprehensive diagnostics of all liver diseases. It is located in Central Jakarta.

Liver Clinic Prof. Ali Sulaiman is a specialized clinic for the treatment and care of all types of liver diseases. The clinic offers comprehensive diagnostics of all liver diseases. It is located in Central Jakarta.
The clinic was founded and directed until his death in 2021 by Prof. H. Ali Sulaiman, M.D., Ph.D., Sp.PD-KGEH, a lecturer in internal medicine and expert in liver diseases with more than 40 years of experience in treating all types of acute and chronic liver diseases.
Since 2021, the Liver Clinic has been led by Dr. dr. Andri Sanityoso Sulaiman, Sp.PD-KGEH. The clinic has specialized staff and facilities that ensure accurate diagnosis of patients with liver disease and provide state-of-the-art care and an effective and appropriate course of treatment depending on the liver disease. Dr. dr. Andri is part of the Hepatobiliary division, Internal Medicine Department, at the University of Indonesia (UI).
The “Liver Clinic” coordinates all liver treatments and offers patients all the different treatment options supported by the Foundation. The credo of Liver Clinic Prof. Ali Sulaiman is: “Our goal is to live a healthy life, free from all kinds of liver diseases. This is the only purpose to achieve a vibrant and happy life.”
HEPADUA® Mini-Liver Implant
A new treatment method for chronic liver disease and liver cirrhosis
HEPADUA® Mini-Liver Implant can save lives
Alcoholic liver diseases, hepatitis B and C lead to liver cirrhosis
In 2010, alcohol-related cirrhosis was responsible for 493,300 deaths worldwide. Other diseases such as hepatitis B (HBV) and C (HCV) and non-alcoholic fatty liver disease (NAFLD) contribute to this problem. The unified response of liver tissue is cirrhotic change, leading to severe clinical symptoms and eventual death.
Millions of people with cirrhosis suffer needlessly and die
159 million people with liver cirrhosis would need a total organ transplant. A complete liver transplant is the only way to definitively treat cirrhosis.
Worldwide shortage of donor liver organs
The waiting list for liver organs is very long and patients often die before a transplant. Effective treatment at an earlier stage of cirrhosis could increase life expectancy and save many lives. HEPADUA® Mini Liver Implants use autologous cells (the body’s own cells) and are not affected by liver organ deficiency.
Production of mini organ implants can save lives
Once clinical research is successfully completed with ongoing clinical trials for the treatment of severe chronic cirrhosis with HEPADUA® Mini Liver Implants, the results will provide the regulatory basis to make HEPADUA® Mini Liver available to a broad range of patients.
HEPADUA® Mini-Liver Implant as a novel treatment for chronic liver disease and liver cirrhosis
Baermed Research specializes in tissue engineering and the development of mini-organs and cell matrix implants. The central goal of Baermed research is to be able to accompany and help patients with chronic liver disease and liver cirrhosis in the long term by means of a living cell transplantation method.
The successful research results of the first clinical trial for the treatment of severe, chronic liver cirrhosis with HEPADUA® Mini Liver Implants encouraged Prof. Baer and his team to intensify clinical research.
“We are very optimistic that with our method of liver cell transplantation – HEPADUA® Mini-Liver Implant – we will find a way to effectively treat patients with liver cirrhosis to improve their quality of life in the long term.”
Prof. Dr. med. Hans U. Baer, Leitung Baermed Forschung

Research Goal
Baermed Research specializes in tissue engineering and the development of mini-organs and cell matrix implants. The central goal of Baermed’s research is to be able to sustainably accompany and help patients with liver cirrhosis by means of a living cell transplantation method. For this purpose, the HEPADUA® Mini-Liver Implant project was launched in 2002.
Basic research
The basic research for the HEPADUA® Mini-Liver Implant, which started 16 years ago, has progressed to the point where Phase II clinical research could begin in March 2022. Phase I clinical research, which began in 2018, was successfully completed in 2020.
Clinical research
The successful research results of the first clinical study (phase I) for the treatment of severe, chronic liver cirrhosis with HEPADUA® mini liver implants encouraged Prof. Baer and his team to intensify clinical research. The second clinical study (phase II) was continued – after a research pause due to the Corona pandemic – in March 2022.
Wide application
Once clinical research is successfully completed with ongoing clinical trials for the treatment of severe chronic cirrhosis with HEPADUA® Mini Liver Implants, the results will provide the regulatory basis to make HEPADUA® Mini Livers available to a broad range of patients.
Results of Baermed Matrix® research

Hepatocyte Matrix Implant Method
Three-dimensional PLLA matrices are fabricated as a support material or spongy scaffold to facilitate effective settlement of seeded cells of different types, in the case of mini-liver of hepatocytes and pancreatic cells. Most of the seeded cells adhere to the PLLA base material, grow, signal, and interact with other cells while maintaining the cells’ metabolic function.
The PLLA scaffold is biocompatible and self-biodegradable. It is produced in the form of a button-like matrix with a diameter of 20 mm. The individual liver and pancreas cells are obtained from tissues removed from the patient. They are mixed (the ratio of cells is evaluated according to cell counting) and arranged to be seeded onto the matrices at a concentration of 1-2 million cells per matrix and incubated for at least 48 hours at 37 °C and 5% CO2.
After incubation, the remaining cells that do not adhere to the matrices are counted and cell viability is assessed, again using the hemocytometer under inverted microscopy with the trypan blue exclusion method. The ratio of hepatocytes to pancreatic cells is patent.
Cell growth can be promoted by growth factors or growth factors that stimulate hepatocytes can be used without implanting pancreatic cells.
Adaptation and improvement of the PLA matrix
Studies on the adaptation and improvement of the PLA matrix were performed in collaboration with the Tarumanagara Human Cell Technology Laboratory (THCT Lab) and the Life Sciences and Facility Management, Wädenswil, Zurich of ZHAW (Prof. U. Graf).
Coated matrices were investigated, and it was found that collagen-coated PLA matrices as a base material led to greater liver cell attachment. These improved bio-cell matrix implants were used in the phase II clinical trial.
Literature
Hendrawan S, Bono E, Hutter A, Weber U, Lheman J, Baer HU. Evaluation of 3D PLLA scaffolds coated with nano-thick collagen as carrier for hepatocytes. J Biomed Mater Res – Part B Appl Biomater. 2020;(March):1-10.
Hendrawan S, Lheman J, Weber U, Baer HU. Hepatocyte and Islet Cell Cotransplantation on Poly-L-Lactide Matrix for the Treatment of Liver Cirrhosis. International Journal of Hepatology. 2020 Oct 13; 2020
Results of Clinical Research Phase I
The results of clinical research are based on scientific work of over ten years.
The specific data and results of the Phase I clinical trial were published by our research group in 2009 and 2018. The safety as well as feasibility of clinical application has been scientifically proven.
Summary of the results
- The average survival rate at one year after transplantation was 75% for all patients.
- The average survival rate at one year after transplantation was 75% for all patients.
- Among patients with a MELD score <= 10, the one-year survival rate was 91%.
- The mean MELD score remained constant in 37 patients for whom 12- or 24-month follow-up data were available. This is in contrast to the literature, which reports a significant worsening of the MELD score in conventionally treated patients.
- In the majority of patients, liver-related blood parameters remained stable or improved 12 months after treatment, with the exception of gamma-GT, which did not improve in most cases.
- The majority of patients also reported improved quality of life one year after treatment.
Literature
A Schwarz, T Lindl, C Höhneke, M Stange, W Pieken. Human Autologous Liver Cell Transplantation for the Treatment of Cirrhosis. The Internet Journal of Gastroenterology. 2009 Volume 10 Number 1
Baer HU, Hendrawan S, The S, Lelosutan SA, Salim G, Lindl T, et al. The Intracorporeal Autologous Hepatocyte Matrix Implant for the Treatment of Chronic Liver Disease: A Modified Clinical Phase I Study. World J Surg Res. 2018; 1067
Testimonial: Maria Handriany
Ms. Maria Handriany was a patient in the Phase I clinical trial of the HEPADUA® Mini-Liver Implant using autologous hepatocytes (the body’s own cells). She lived without symptoms or complications and without special treatment. The elderly patient died of old age six years after implantation.
“I suffered from severe liver cirrhosis for two years before implantation. After implantation, I noticed that my albumin level did not drop anymore. I also feel fitter and fresher every day.”
Maria Handriany (†)

Patient experience clinical research phase II
In the Phase II clinical trial, 10 patients with chronic liver cirrhosis have been treated with the HEPADUA® Mini-Liver Implant and compared to non-operated patients. This clinical investigation is intended to demonstrate the efficacy of the procedure.
The first two patients treated with the HEPADUA® Mini-Liver Implant in the Phase II study, reported their positive experiences with this novel treatment method during follow-up visits to the Liver Clinic Prof. Ali Sulaiman in Jakarta.
They says that treatment with the HEPADUA® Mini-Liver Implant has significantly improved their life. Since the operation, they are in good shape and can again actively participate in social life.
For more information on the results of the Phase I clinical trial, please contact the principal investigator, dr. Barlian Sutedja, Sp. B. via Clinical Research Officer Suryadi The, MD(see Team Research).

